Molecular and Cellular Mechanisms of Pain Hypersensitivity
Background
Chronic pain is the leading cause of long-term disability and suffering in humans. Common chronic pain conditions include headache, low back pain, cancer pain, arthritis, and neuropathic pain. Long-lasting sensitization of the peripheral and central nociceptive circuits is one of the main underlying causes of pain hypersensitivity in chronic pain conditions. Alterations in primary sensory neurons, spinal neurons, and non-neuronal cells contribute to the long-lasting sensitization, which rely on de novo gene expression to support biochemical and structural reorganization of the pain pathway.Gene expression can be modulated at different steps, including transcription, RNA splicing, mRNA translation, and post-translational modification of proteins. Most studies hitherto have focused on transcriptional regulation; however, recent work has shown that the abundance of proteins in the cell is determined predominantly by the rate of mRNA translation. Inhibition of mRNA translation alleviates chronic pain in animal models; however, the molecular and cellular mechanisms underlying the beneficial effect of translation inhibitors remain elusive.
Research
We aim to decipher mechanisms by which mRNA translation promotes pain sensitization by identifying mRNAs mediating these phenomena and studying their function. We study the role of mTORC2-dependent structural plasticity in peripheral and central nociceptive circuits and investigate the role of neuroimmune interactions in the development of pain hypersensitivity. We also investigate the role of gut microbiome in fibromyalgia, a chronic pain condition.In addition, we are interested in mechanisms underlying the modulation of spinal perineuronal nets in pathological pain and study the heterogeneity of spinal projection neurons. Our research is directed toward identification of novel molecular targets and ultimately developing better therapeutics to relieve pain and improve quality of life of individuals living with pain.
Mechanisms underlying brain disorders
Background
Regulation of cellular proteome at the level of mRNA translation is essential for normal brain function, and dysregulation of translational control might cause neuropsychiatric disorders. Deviations from the optimal level of synaptic protein synthesis can lead to impaired synaptic circuitry, and may contribute to the development of cognitive deficits and autistic traits. Several brain disorders, with high rates of autism, are associated with loss of function of translational repressors. Mutations in TSC1/2, PTEN and FMR1 genes cause Tuberous Sclerosis, PTEN hamartoma syndrome and Fragile X syndrome, respectively and lead to intellectual disability, autistic-like behavior, epileptic seizures and language impairment. Despite the prevalence of these neuropsychiatric disorders, there is a slow progress in understanding the pathophysiology and development of effective therapies.
Research
We study the role of translational control in synaptic plasticity and memory formation, and investigate how aberrant translation leads to neuropsychiatric disorders such as fragile X syndrome and autism. We use a multidisciplinary approach combining electrophysiology, behavioral analysis, imaging and genome-wide transcriptional/ translational profiling to decipher the molecular underpinnings of these disorders.The lab currently has projects in the following topics:
Pain
Memory formation and brain disorders
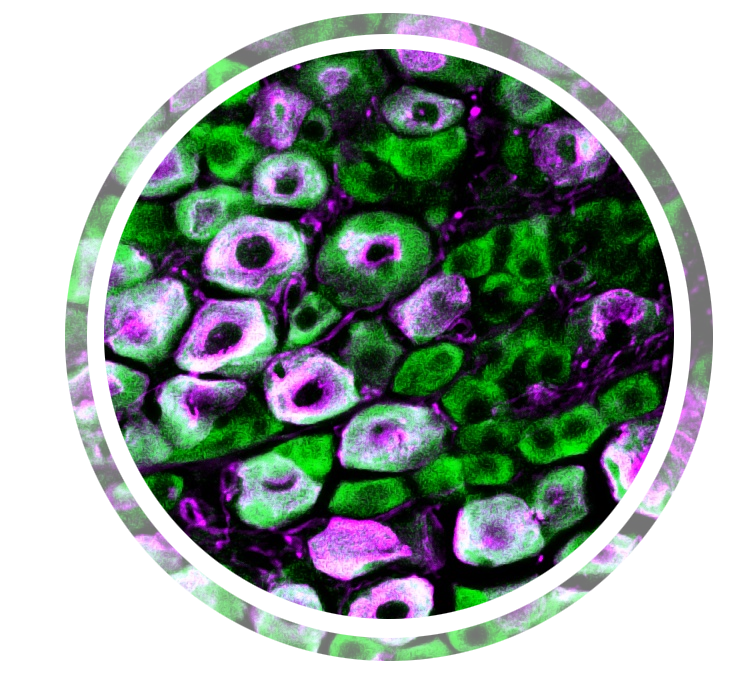
- Genome-wide and cell type-specific translational profiling in chronic pain
- Investigating the role of mTORC2 in pain plasticity
- Modulation of extracellular matrix/PNNs in neuropathic pain
- Heterogeneity of spinal projection neurons
- The role of the gut microbiota in fibromyalgia
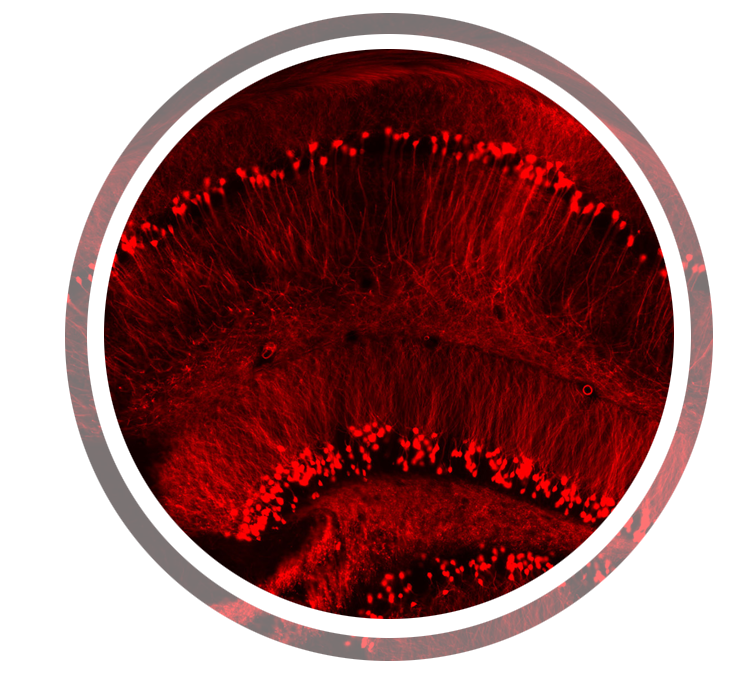
- p-eIF2a in fragile X Syndrome
- Translational control in non-neuronal cells in memory
- Cell type-specific mechanisms of eIF2a-dependent translational control in learning and memory






